LivaNova PLC (Nasdaq: LIVN), a leader in medical devices, today announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) and CE Mark for its Essence™ In-Line Blood Monitor (ILBM) (ILBM), which enables perfusionists to take accurate and continuous measurements of important blood parameters during cardiopulmonary bypass (CPB). The ILBM is integrated into LivaNova’s next-generation CPB platform, the Essenz™ Perfusion System. Perfusionists can thus access and manage reliable blood parameters directly from the system cockpit without the need for additional monitors or mounts.
This press release contains multimedia content. View the full announcement here: https://www.businesswire.com/news/home/20230830618951/en/
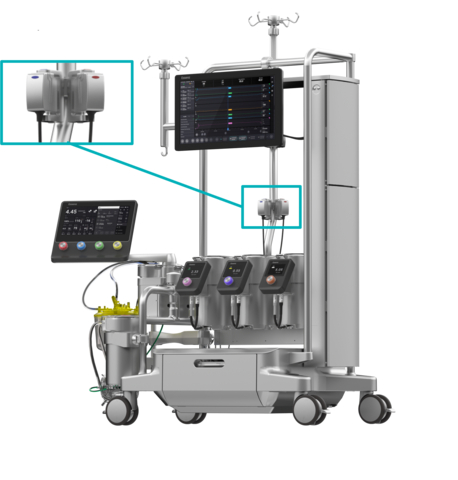
Essenz In-Line Blood Monitor shown with the Essenz Perfusion System (Photo: Business Wire)
Existing blood gas analyzers only reflect the clinical condition of a patient at the moment the sample is taken. However, this condition can change quickly and the results are no longer relevant.1.2 With the Essenz ILBM, perfusionists receive continuous inline monitoring of patient parameters over the entire duration of a procedure. This allows for a patient-tailored perfusion approach based on data-driven decisions.
“Dynamic conditions can rapidly alter a patient’s blood parameters during cardiopulmonary bypass surgery,” said Marco Dolci, President of LivaNova, Cardiopulmonary. “The Essenz In-Line Blood Monitor enables continuous monitoring throughout the patient’s procedure. Having access to precise, real-time measurements directly from the Essenz Perfusion System allows quick decisions to be made and individual treatment strategies to be developed for the patient.”
Based on proven B-Capta™ sensor technology, the Essence ILBM is the only in-line blood analysis system that operates within the Clinical Laboratory Improvement Amendments (CLIA) guidelines, providing parameter values consistent with hospital blood gas analyzers, and even before the comparison.3 To enable accurate monitoring, the ILBM provides measured values for oxygen saturation, hematocrit, oxygen partial pressure, and temperature instead of calculated values for these parameters.
In addition, the Essence ILBM does not need to be calibrated to set up the device measurements, which saves the perfusionist time when setting up the device, which is particularly important in emergencies. Arterial and venous parameters are automatically transmitted to the Essenz™ Patient Monitor, supporting data-driven decision making and implementation of Goal-Directed Perfusion (GDP), a therapy that effectively reduces the risk of acute kidney injury.4 The latest software for the heart-lung machine, version 1.3, integrates the ILBM with the Essence Perfusion System and is designed to continuously improve the user experience.
The Essenz Perfusion System is based on 50 years of a trusting partnership and was designed and developed in cooperation with over 300 customers worldwide. It includes a next-generation heart-lung machine (HLM), a patient monitor, and precise sensor technology that now includes the ILBM. The Essence Perfusion System is currently available in Europe, USA, Canada, Australia, Japan and United Arab Emirates. Since its market launch in February 2023, more than 1,000 patients have been cared for with this system worldwide. For more information on the entire Essence Perfusion System, visit the LivaNova website.
Watch the Essenz Perfusion System brand video here.
*Note: The Essence HLM is not available for sale in all countries. Visit the LivaNova website for important safety information.
credentials
-
Ottens J et al. Improving Cardiopulmonary Bypass: Does Continuous Blood Gas Monitoring Have a Role to Play? – JECT. 2010;42:191-198 -
Trowbridge CC et al., The Effects of Continuous Blood Gas Monitoring During Cardiopulmonary Bypass: A Prospective, Randomized Study-Part II, The Journal of Extracorporeal Technology, 2000 -
Perfusion, 2022, Vol. 0(0) 1–7, Marloes van Hoeven, Eddy Overdevest, Joyce Curvers, Henri van Heugten: A comparison of continuous blood gas monitors during cardiopulmonary bypass Livanova B-Capta, Terumo CDI 500, spectrum medical M4 -
Goal-Directed Perfusion to Reduce Acute Kidney Injury: A Randomized Trial Ranucci M. et al.
J Thorac Cardiovascular Surg. 2018 Nov; 156(5): 1918-1927.e2. doi.org/10.1016/j.jtcvs.2018.04.045
About LivaNova
LivaNova PLC is a global medical technology company built on nearly five decades of experience and an unwavering commitment to bring hope to patients and their families through medical technologies and to provide life-changing improvements for the mind and heart. Headquartered in London, LivaNova employs around 2,900 people and has a global presence in more than 100 countries, serving patients, healthcare professionals and healthcare systems. Visit www.livanova.com for more information.
Safe Harbor Declaration
This press release contains “forward-looking statements” regarding the Company’s objectives, beliefs, expectations, strategies, intentions, plans and underlying assumptions, as well as other statements that are not necessarily historical facts. These statements include, but are not limited to, statements about the Essenz Perfusion System, the Essenz HLM, the Essenz Patient Monitor, and the Essenz ILBM. Actual events could differ materially from those discussed in the forward-looking statements due to a number of factors, including those factors discussed in Item 1A of the Company’s most recent Annual Report on Form 10-K, supplemented by any risk factors discussed in the quarterly reports on Form 10 -Q and the current reports on Form 8-K. LivaNova undertakes no obligation to update the information contained in this press release to reflect subsequent events or circumstances.
The source language in which the original text is published is the official and authorized version. Translations will be included for a better understanding. Only the language version that was originally published is legally valid. Therefore, compare translations with the original language version of the publication.
View original version on businesswire.com: https://www.businesswire.com/news/home/20230830618951/en/
Selected leveraged products on Livanova PLC
With knock-outs, speculative investors can participate disproportionately in price movements. Simply select the desired lever and we will show you suitable open-end products on Livanova PLC
Leverage must be between 2 and 20