BioNTech and Pfizer have received approval in the USA for booster shots, i.e. the third vaccination for five to eleven year olds. Other than that, there was little good news. On the contrary, the EU postponed deliveries planned for the period June to August. No wonder there is hardly any vaccination on the continent, especially since almost all restrictions have been lifted. One wants to be prepared for the fall, so that more vaccine will be ordered again from September, but nobody can say at the moment how exactly the situation will be then.
The situation is complicated by the fact that there are still no results on the adapted vaccines. Because depending on how these turn out, governments will want a lot of the new substance – or push the population to refresh themselves with the tried-and-tested vaccine, which, however, is already working rather poorly.
Meanwhile, the number of cases in the United States is increasing. Almost 60 percent more infections were detected compared to two weeks ago. The stock market is also watching closely to see whether the wave can be slowed down with the generous use of Pfizer’s drug Paxlovid. In any case, the prices of many vaccine manufacturers have risen again in the past few days, which may also be due to the favorable valuations.
Notice of Conflicts of Interest:
The majority owner of the sole shareholder of Finanzen Verlag GmbH, Mr. Bernd Förtsch, has taken direct and indirect positions on the following financial instruments mentioned in the publication or related derivatives that can benefit from any price development resulting from the publication: BioNTech, CureVac, GlaxoSmithKline , Novavax, Pfizer, Valneva.
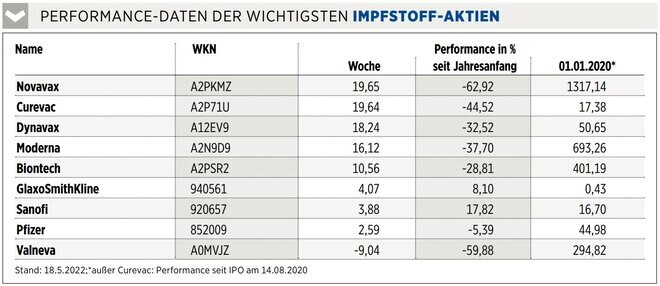
INVESTOR INFO
The EU wants to terminate its purchase contract for the COVID vaccine because approval has still not been obtained. This is a heavy blow, the EU was Valneva’s most important customer with 60 million agreed doses alongside the British who had already jumped off. Valneva now has until Pentecost to either get approval or agree on an alternative plan. The European Medicines Agency has at least accepted Valneva’s application for approval. If the okay comes, the price should recover. Otherwise, it will probably result in further losses, the stock is still worth considerably more than before the pandemic.
It is not easy to explain why the adjuvant manufacturer Dynavax is rising in the week when Valneva, one of its most important customers, is announcing rather bad news. Investors may be betting that China will soon be vaccinated more given the problems with controlling the number of infections – and that the vaccine from Clover Biopharmaceuticals, another Dynavax customer, will be approved. However, there is no news about this. Dynavax expects the launch of its hepatitis B vaccine in Europe in the coming weeks and phase 1 data on the shingles vaccine in the second half of the year. Speculative.
Leverage must be between 2 and 20
No data
More news about BioNTech (ADRs)
Image sources: peterschreiber.media / Shutterstock.com, Zoran Mircetic/iStock, Finanzen Verlag
ttn-28




